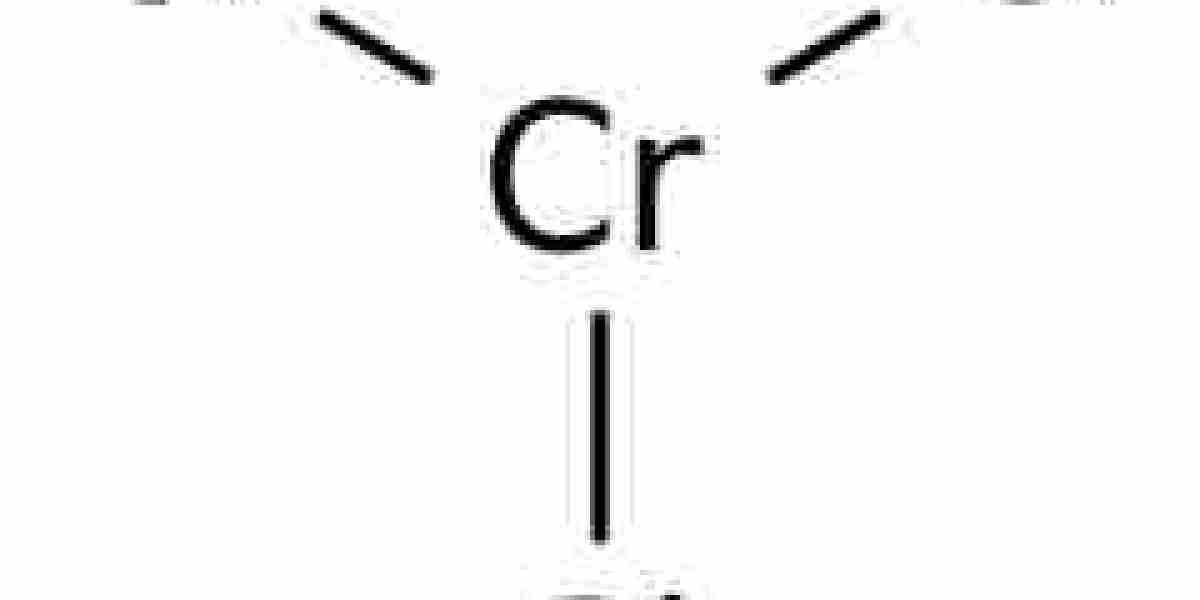
Chromium Chloride(CrCl3)
Numerous studies have been conducted to evaluate the safety of trivalent chromium. In a comparative evaluation, toxicities of chromium picolinate, niacin-bound chromium and CrCl3 were examined. At equal and physiological relevant doses, chromium picolinate demonstrated mutagenic potential at the hypoxanthine (guanine) phosphoribosyltransferase locus in Chinese hamster ovary cells, while at the same dose CrCl3 and niacin-bound chromium exhibited no mutagenicity. In another related study, chromium picolinate was found to produce chromosome damage in Chinese hamster ovary cells 3- to 18-fold greater than control levels for soluble doses of 0.050, 0.10, 0.50, and 1.0 mM after 24-hour treatment, while no chromosome damage was observed following treatment with niacin-bound chromium or nicotinic acid. The cause of damage was inferred to be due to the picolinate ligand since picolinic acid alone resulted in chromosomal damage and is clastogenic. Although a number of published studies demonstrated the toxicity including potential neurological and other deleterious effects of chromium picolinate, however, it received self-affirmed GRAS (Generally Recognized as Safe) status recently.
A large body of evidence reveals enhanced bioavailability, efficacy, and safety for niacin-bound chromium. Based on the results of extensive safety studies including acute oral, acute dermal, primary dermal, and eye irritation studies, 90-day sub-chronic toxicity, Ames’ bacterial reverse mutation assay and mouse lymphoma mutagenicity assay, niacin-bound chromium received self-affirmed GRAS status.
No significant toxic effects of CrCl3 have been reported. No detailed safety studies have been reported on other trivalent chromium complexes. Additional studies using different sources and formulations of chromium, for example brewer's yeast (23.3 µg Cr/day) and CrCl3 (200 µg Cr/day), have been reported. These studies evaluated the effect of the added nutritional supplement on glucose tolerance, serum lipids, and antidiabetic drug dosage in a 16-week, randomized, double-blind, crossover trial that included 78 patients with type 2 diabetes. Both forms of chromium supplementation resulted in significant decreases in glycemia as assessed by mean fasting and 2h glucose, and fructosamine, a marker of short-term protein glycation. Additional studies have been reported for jiangtangkang (8 g t.i.d.), a chrysanthemum product high in chromium, on glucose and insulin metabolism in 188 patients with type 2 diabetes. After 2 months, jiangtangkang treatment reduced fasting and postprandial blood glucose and HbA1c without any corresponding change in plasma insulin. A 16-week, double-blind, randomized, crossover trial of CrCl3, brewer's yeast that contained chromium as GTF, brewer's yeast extract without GTF, and a placebo was conducted in 43 patients with diabetes. This study was interesting in that although positive effects of chromium on glucose and insulin metabolism were not observed (specifically, fasting glucose and the glucose response to either a standard meal or tolbutamide were not significantly altered by any of the treatments), ketosis-resistant patients experienced a significant increase in postprandial insulin after treatment with the brewer's yeast that contained GTF.