Oxidation by Chromic Acid
One of the reagents that is commonly used for oxidation in organic chemistry is chromic acid. This reagent is straightforward to use once deciphered. However, there are a vast number of different ways that textbooks (and instructors) show it being used in reactions. Chromic acid, H2CrO4 , is a strong acid and is a reagent for oxidizing alcohols to ketones and carboxylic acids. For reasons primarily concerning safety and convenience, chromic acid tends to be produced in a reaction vessel as needed (through the addition of acid to a source of chromium), rather than being dispensed from a bottle.
This is where the trouble begins. Choosing a source of chromium to produce H2CrO4 is a lot like choosing a favorite brand of bottled water. Beyond the packaging, they’re pretty much all the same. Depending on which textbook or instructor you have, however, you might see several different ways to do this, and it can be very confusing.
The key point is that Na2CrO4 (sodium chromate), Na2Cr2O7 (sodium dichromate), KCrO4 (potassium chromate), K2Cr2O7 (potassium dichromate), and CrO3
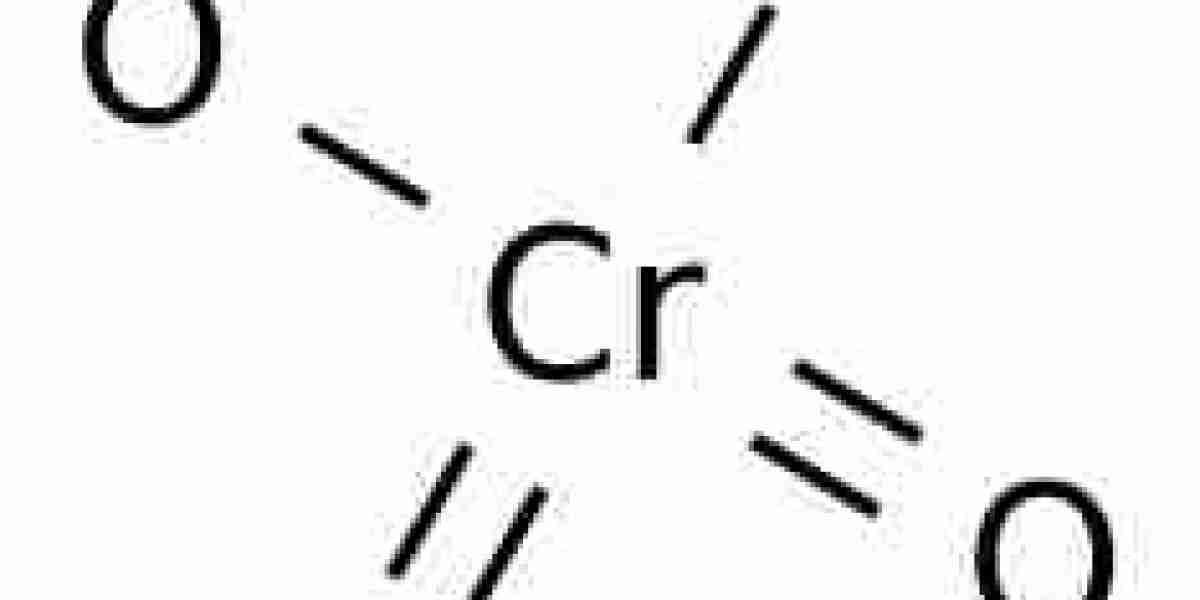
(chromium trioxide) are alike in one crucial manner: when they are combined with aqueous acid, each of them forms H2CrO4, and ultimately it is H2CrO4 that is involved in the important chemistry. Unfortunately I rarely see this point explained in textbooks. I remember this causing some confusion for me when I took the course. The K or Na ions present are just spectators. Once H2CrO4 is formed, its reactions are pretty straightforward: it converts primary alcohols (and aldehydes) to carboxylic acids and secondary alcohols to ketones.
The reverse reaction occurs when molecular acid is dehydrated. This is often what happens when concentrated vitriol is added to a dichromate solution. The colour changes from orange to red (chromic acid). Chromium trioxide crystals precipitate from the mixture, but their colour does not change further. LMCT transitions result in the colours. Chromium trioxide is anhydride of molecular acid. It is a Lewis acid and can react with Lewis bases. Pyridine reacts in non-aqueous media. An adduct of chromium trioxide and pyridine, Collins reagent can be used in a wide variety of oxidations. During its oxidation of alcohols in aqueous solutions, it (H2CrO4) makes chromic ester. During this reaction, the oxygen atom bridges the carbon and chromium atoms.